MKdir

RESEARCH

Our research group is dedicated to advancing the field of catalysis and material science through our unique 3G strategy. This approach encompasses three critical stages:
Edge 1 (Science): We embark on our journey with a fundamental perspective, where initial material and catalyst designs are conceptualized. These designs are based on promising theoretical performance indicators, setting the groundwork for practical applications.
Bridge (Applied Science): Building upon the initial designs, we conduct rigorous experiments to evaluate their performance. The outcomes of these experiments serve as valuable feedback for our computational team, enabling them to refine and enhance the designs further.
Edge 3 (Engineering): In the final phase, we focus on the practical application of our research. Utilizing the optimized material designs, we develop proof-of-concept catalysts aimed at demonstrating real-world efficacy.
Guided by our 3G strategy, our goal is to develop fundamentally sound catalysts and materials that not only showcase innovative scientific principles but are also tailored for industrial applications.

Methane Direct Conversion
to Value Added Products

Developing catalysts that can efficiently and selectively transform light alkanes to value-added products would have a transformative effect on the utilization of hydrocarbon resources and has long been a goal of catalysis research. The increased availability of shale gas resulting from hydraulic fracturing has also stimulated recent interest in designing catalytic processes for the partial oxidation of alkanes.

Desulfurization

Hydrogen energy has been drawing wide attention since it can avoid CO2 emission triggering the global warming. To satisfy the needs of hydrogen energy resources, the hydrogen productions must be quantitatively increased and diversified. Petroleum cokes consist of various chemicals including carbon, hydrogen, sulfur, etc., and can produce hydrogens by gasification. In order to employ hydrogens from gasified petroleum cokes, the impurities such as COS and H2S must be removed first. This is because the impurity of sulfur can deactivate the hydrogen fuel cell catalysts. Therefore, the desulfurization (removal of COS and H2S) from gasified petroleum cokes is a critical step to generate high purity hydrogens.

Electro Chemistry
(OER, ORR, HER)
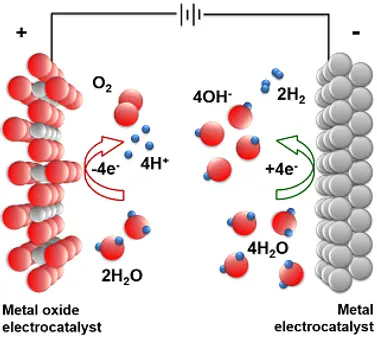
Oxygen Evolution Reaction (OER):
The Oxygen Evolution Reaction (OER) is crucial for energy storage and conversion, such as in water electrolysis. It converts water into oxygen, requiring efficient catalysts to overcome its high energy demands. Our research seeks to develop materials that enhance OER efficiency, promoting sustainable energy solutions.
Oxygen Reduction Reaction (ORR):
Critical for fuel cells and metal-air batteries, the Oxygen Reduction Reaction (ORR) converts oxygen to water or hydroxide ions. The challenge lies in achieving fast reaction rates with minimal energy loss. We focus on creating advanced catalysts that improve ORR activity and stability, driving forward clean energy technologies.
Hydrogen Evolution Reaction (HER):
The Hydrogen Evolution Reaction (HER) is key for producing hydrogen fuel through water electrolysis, demanding catalysts that operate with low energy input. Our efforts are directed towards discovering effective, durable, and cost-efficient electrocatalysts for HER, facilitating the transition to hydrogen-based energy.

Chemical Looping Combustion

The dramatic increase in shale gas production from hydraulic fracturing has increased usage in fossil fuels to generate electricity and it leads to significant increases in CO2(g) emission. Chemical looping combustion technology (CLC) emerged as a suitable technology that can efficiently and eco-friendly generates electricity from fossil fuel; CLC can achieve low NOx emission and inherent CO2 capture since fuel, mainly CH4(g), does not directly contact with air. Because of the above reasons, Chemical looping combustion technology has been widely studied and developed.

De NOx

Nitrogen oxides (NOx) emitted from the combustion of fossil fuels have been considered the primary cause of air pollution, which is not only toxic by itself but also causes photochemical smog, acid rain, ozone layer depletion, and resulting global warming [1,2]. In the face of rising NOx emissions, regulations implemented by governments have been strengthened, and accordingly, there has been a lot of attention on NOx removal technologies.

Methane Pyrolysis
(Molten Salts)
